Tailoring Personalized Dosage Regimens of NMN Based on NAD Concentration
Introduction
Nicotinamide mononucleotide (NMN), one precursor of nicotinamide adenine dinucleotide (NAD+), has been ascertained to be implicated with multiple biological processes such as cellular redox regulation and metabolism as well as DNA repair. Herein, post-hoc analysis of a double-blinded clinical trial is carried out. On the premise of safety, to optimize NMN utilization, personalized dosage regimen can be developed by monitoring the NAD concentration.
Research protocol
A total of 80 healthy middle-aged adults (age: 40 to 65) are enrolled in the randomized, double-blinded, controlled clinical trial of NMN supplementation, who are randomly assigned into four groups and administrated with placebo or NMN (300 mg, 600 mg, or 900 mg) for 60 days. The clinical data including age, sex, body mass index (BMI), blood biological age, homeostatic model assessment for insulin resistance (HOMA-IR), blood NAD concentration, 6-minute walk test and 36-item short-form survey (SF-36), along with adverse events, are collected at baseline and after supplement, followed by comparison and correlation analysis.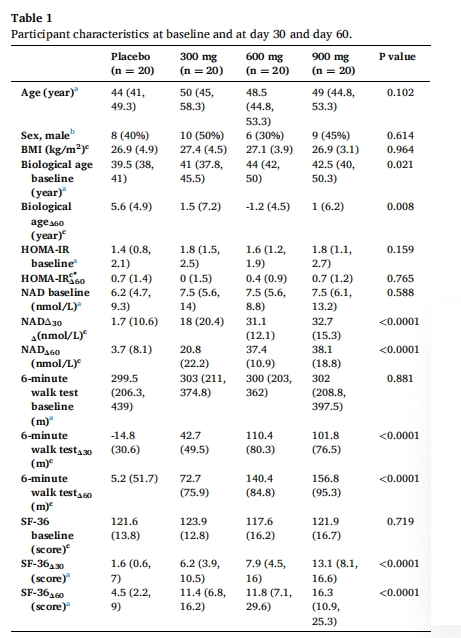
The association of participant clinical data at baseline and after supplement of NMN
NAD concentration change (NADΔ) is dose-dependently increased post NMN supplementation, with a large coefficient of variation (29.2–113.3%) within group. Notably, only HOMA-IR has a prominent association with blood baseline NAD. As a whole, NMN supplementation has a positive impact on the physical endurance and general health conditions of healthy adults, as evidenced by the obvious improvement of six-minute walking distance, blood biological age, and SF-36 score. In addition, the increase of about 15 nmol/L in NADΔ is related to clinically improvements in the walking distance of 6-minute walk test and the SF-36 score.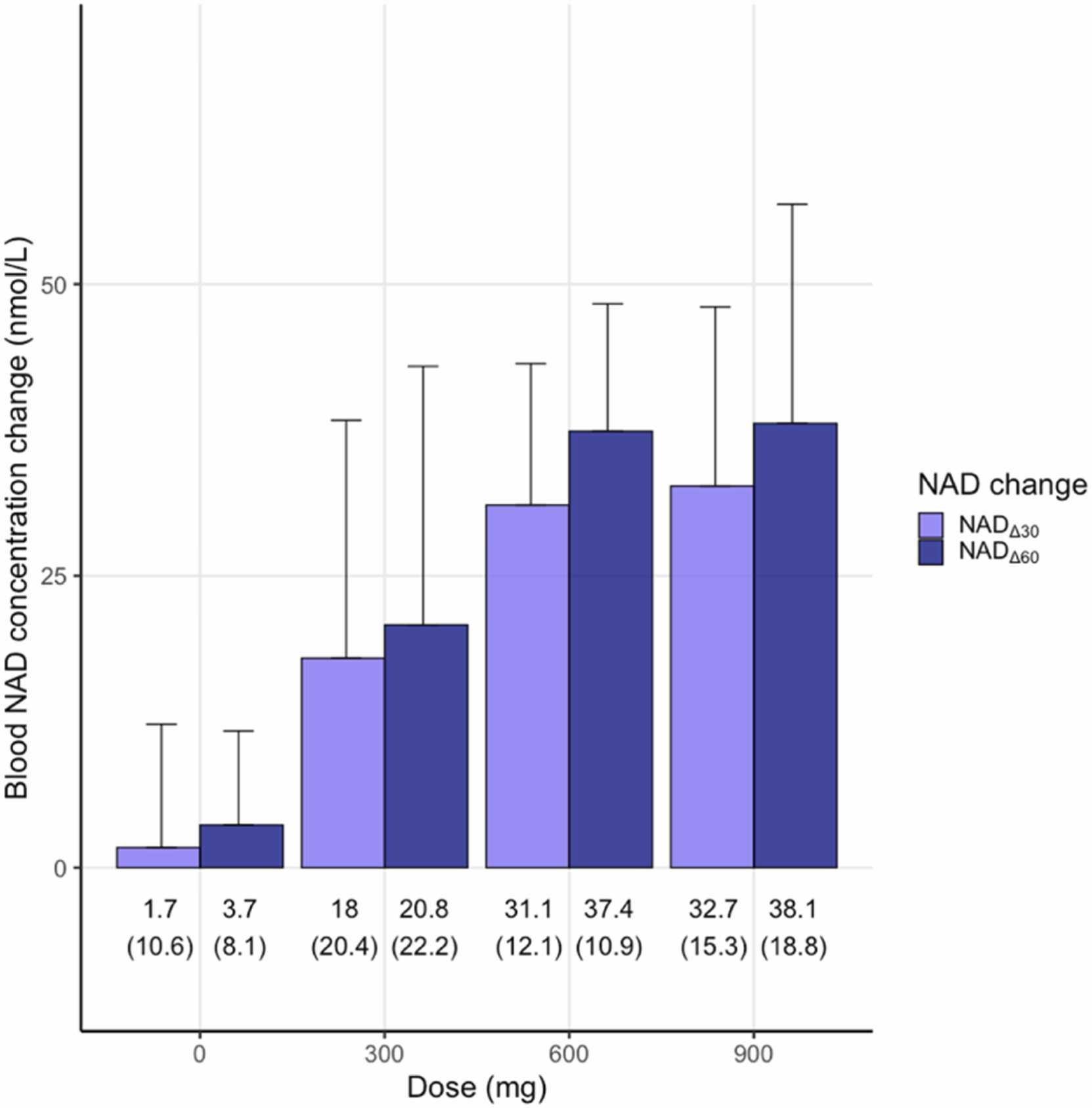
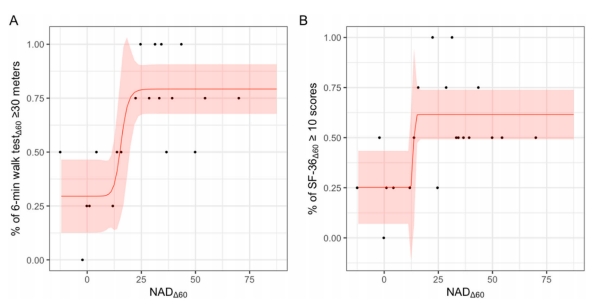
The safety oral dose of NMN in clinical trials
As demonstrated by the registered clinical trials NCT04823260 and CTRI/2021/03/032421, NMN supplementation can boost blood NAD concentration, which is safe and well tolerated with daily oral administration of 900 mg. Strikingly, clinical efficacy expressed by blood NAD concentration and physical performance reaches highest at a dose of 600 mg daily oral intake.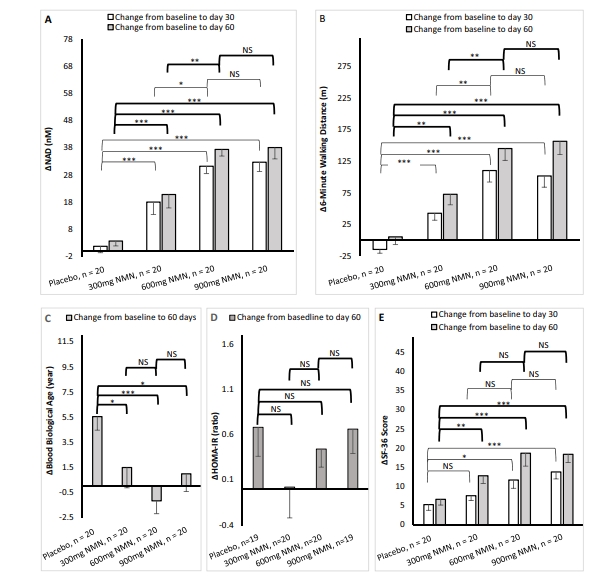
Conclusion
Blood NAD concentration is increased by NMN supplement at a dose-dependent manner. Personalized regimen of NMN supplement should be based on the close monitoring of NAD concentration change. In addition to longer follow-up duration and large sample size, future trials on the efficacy of NMN supplement should pay much attention to the factors affecting baseline NAD concentration.
Reference
[2] Song Q, Zhou X, Xu K, Liu S, Zhu X, Yang J. The Safety and Antiaging Effects of Nicotinamide Mononucleotide in Human Clinical Trials: an Update. Adv Nutr. 2023;14(6):1416-1435. doi:10.1016/j.advnut.2023.08.008
BONTAC NMN
As David Sinclair, a famous professor of genetics at Harvard University, once pointed out in an interview, NMN has unstable molecular structure, which is easily degraded into nicotinamide if stored in the conventional environment. The satisfactory efficacy of NMN cannot be guaranteed if the quality and purity NMN products are not high.
BONTAC is the first choice of NMN raw material suppliers worldwide, who has dedicated to the manufacture of raw material for enzyme and natural products for 12 years, with self-owned factory, 173 patents and professional R&D team. The purity of BONTAC NMN can reach up to 99.5%. Also, BONTAC is the NMN raw material supplier of David Sinclair team, who uses the raw materials of BONTAC in a paper titled “Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging”. Our services and products have been highly recognized by global partners. The coenzyme products of BONTAC are widely used in fields such as nutritional health, biomedicine, medical beauty, daily chemicals and green agriculture.
Disclaimer
This article is based on the reference in the academic journal. The relevant information is provided for sharing and learning purposes only, and does not represent any medical advice purposes. If there is any infringement, please contact the author for deletion. The views expressed in this article do not represent the position of BONTAC.
Under no circumstances will BONTAC be held responsible or liable in any way for any claims, damages, losses, expenses, costs or liabilities whatsoever (including, without limitation, any direct or indirect damages for loss of profits, business interruption or loss of information) resulting or arising directly or indirectly from your reliance on the information and material on this website.
